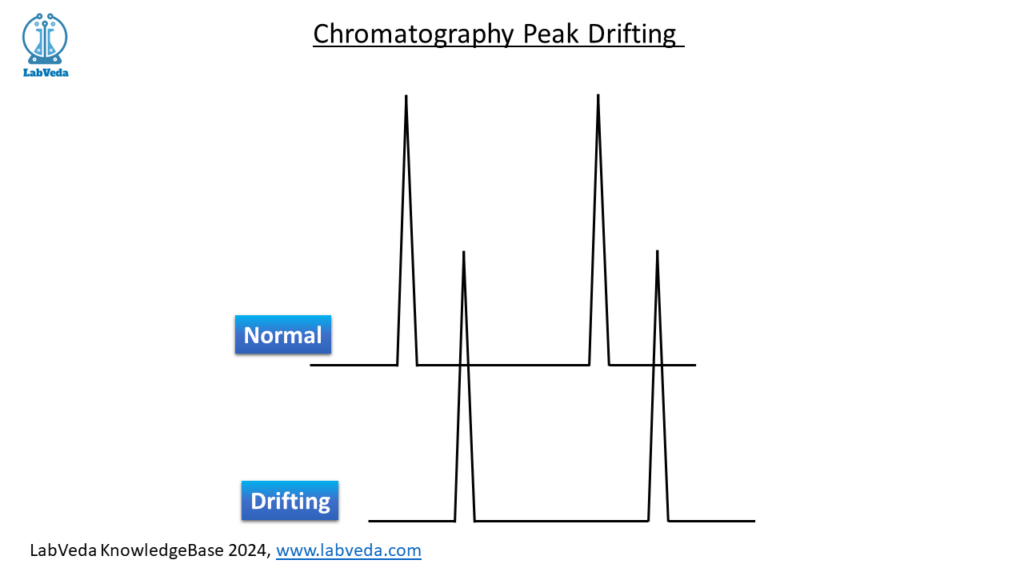
A drift in analyte retention time over time is sometimes observed in HPLC chromatograms. This drift takes analytes outside their ‘retention window’ within the data analysis software leading to analyte peaks being missed (Figure 1). Peak drifting is often seen during long analysis sequence, analyzing “dirty” samples, or when installing a new HPLC column. A stable analyte retention time is desired for accurate data analysis.
Reasons for Drifting HPLC Peaks
- Inadequate column equilibration: This is especially true for normal phase columns. Since solubility of water in organic solvents such as hexane is poor, it takes long time for the column to equilibrate. For this reason, solvents that are dry should be avoided and solvents that are “half-saturated” with water can be considered. This can speed up equilibration. Inadequequate equilibration is less of a problem in reversed phase HPLC.
- Strongly adsorbed sample component: Strongly adsorbed component from the sample can change the surface chemistry of the column and it can lead to drifting retention times. This issue is especially observed when running long sequence runs.
- Changing mobile phase composition: If the online mixing is not used then there could be slow evaporation of mobile phase components. This is especially true if the mobile phase is sparged with a gas such as helium.
- Hydrolysis of the bonded phase: The bonded phases are sensitive to pH. Slow hydrolysis of the bonded phase can occur even when mobile phase pH is within the recommended limits. Isocratic conditions are better than gradient conditions to minimize drifting. While hydrolysis occurs in both, the bonded phase adsorbs back to itself and is in local equilibrium. This equilibrium is disturbed when using gradient condition with higher organic solvent content.
- Temperature: Column temperature can impact retention time. If different lab temperatures are used at different times during the day or week then retention times can change.
- Increased backpressure: This could come from a contaminated column or a clogged frits.
- Leaks: A leak can lead to retention time changes. If the leak is especially small then it can go unnoticed.
- Flow rate changes: A malfunctioning pump can produce variable flow. This is not very common cause of retention time drift.
- Sample overloading: Sample overloading can cause peak fronting and it can lead to reduced retention time.
Identification
Retention time drifting can be difficult to detect especially if the samples are being run continuously for a long periods. It is a good practice to run check standards at frequent intervals so that the issue can be easily diagnosed.
Troubleshooting Drifting Peaks
| No. | Factor | How to troubleshoot |
|---|---|---|
| 1. | Inadequate equilibration | Allow sufficient equilibration time; Inject several standard samples to confirm retention time reproducibility |
| 2. | Strongly adsorbed sample components | Consider sample pretreatment; If possible use dilute samples |
| 3. | Changing mobile phase composition | Using online mixing. Keep helium sparging at low levels |
| 4. | Hydrolysis of the bonded phase | Closely monitor mobile phase pH; use isocratic conditions when possible; change column if necessary |
| 5. | Temperature | Keep lab temperatures constant |
| 6. | Increased backpressure | Clean clogged frits or column |
| 7. | Leaks | Check all connections and flow path for possible leaks |
| 8. | Flow rate changes | Calibrate pump performance by measuring accurate flow rate |
| 9. | Sample overloading | Use dilute samples; use higher capacity column |
Summary
HPLC peak drifting is a serious and commonly observed issue especially when running longer campaign runs. It can lead to inaccurate data analysis as samples peaks can be missed due to change in retention time. Drifting is most commonly associated with a dirty sample. Inadequate equilibration time is another common reason. System related issues such as temperature or flow rate changes are less likely to cause the drifting
