Table of Contents
A. Introduction
Chromatography is an important biophysical technique that enables separation, identification, and purification of components of a mixture for qualitative analysis and quantitative determinations. Three components are central to any chromatography system.
- Stationary phase: This phase is composed of a solid phase or a layer of a liquid adsorbed on the surface a solid support
- Mobile phase: This phase is always composed of liquid or a gaseous component
- Analytes: Molecules to be separated or the solutes
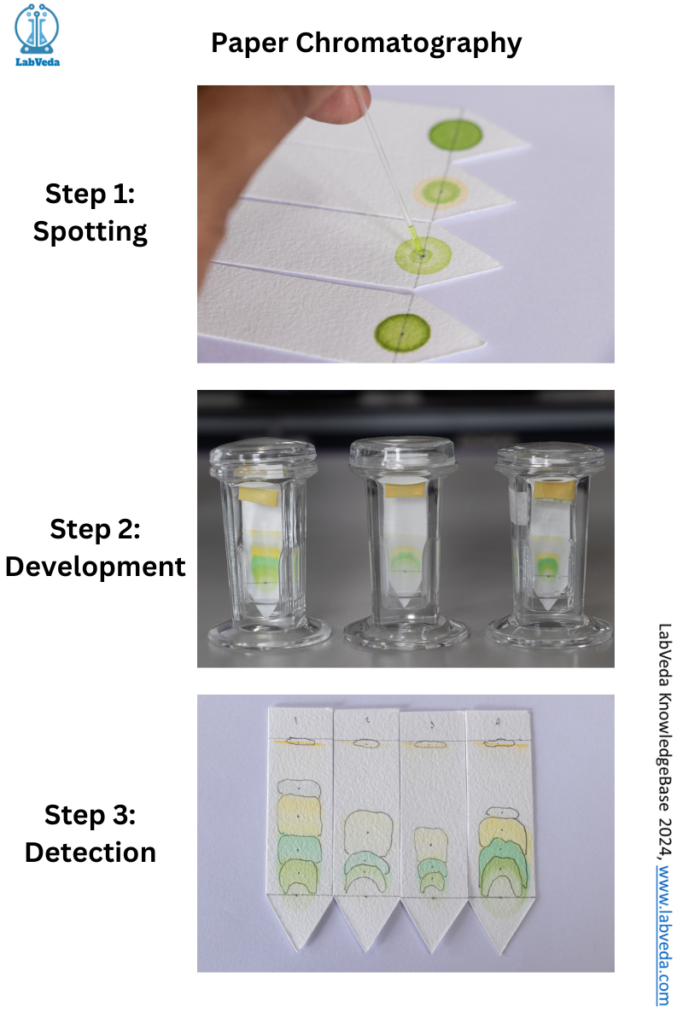
Figure 1 demonstrates chromatography principles using a filter paper as a stationary phase for separation of mixture components. This type of chromatography is called paper chromatography. The key steps involved are as follows.
- Sample loading or spotting on the paper: Using a capillary tube, micropipette, the sample is spotted on the paper at a specific position. This facilitates easy interpretation of the chromatogram.
- Chromatogram development: For this step, the paper is immersed in the mobile phase. The mobile phase moves over the sample on the paper because of the capillary action. The mobile phase carries the mixture components with it.
- Drying of paper and detection of the compound: The paper is dried after the chromatogram. A detecting solution is sprayed on the developed paper and dried thoroughly for the identification of the sample chromatogram spots.
B. Chromatography Principle
The solute partitions between the mobile phase and the stationary phase as it moves across the stationary phase with the mobile phase. The degree to which it get adsorbed or interacts with the stationary phase will determine how fast and how far the solute is carried by the mobile phase. Therefore substances which interact with the solid phase differently can be separated.
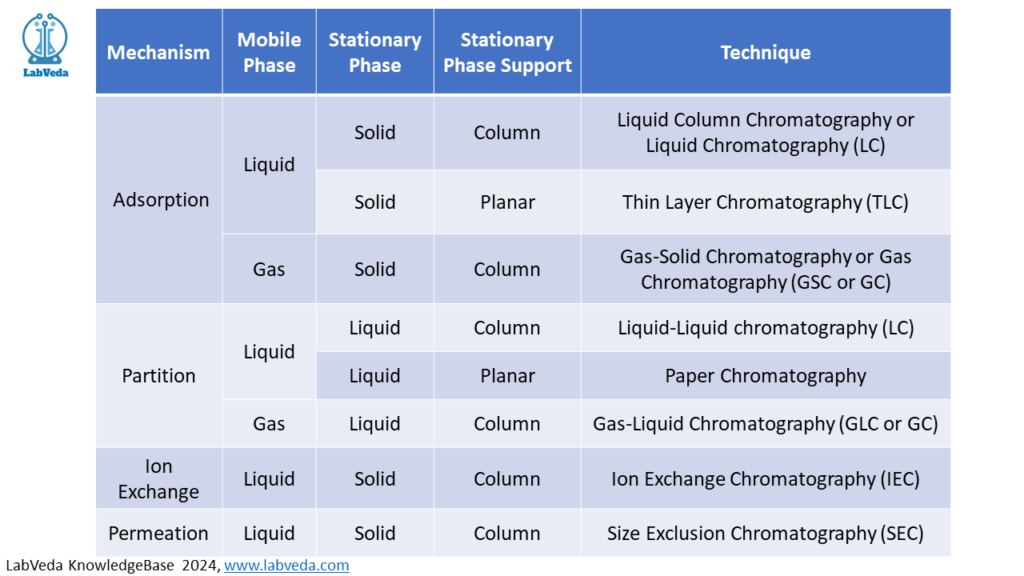
There are 4 major mechanisms responsible for interaction between the analytes and the stationary phase. These are adsorption, partition, ion exchange, and permeation. . Chromatography methods based on partition are very effective on separation, and identification of small molecules as amino acids, carbohydrates, and fatty acids. However, chromatography based on ion-exchange are effective in the separation of macromolecules as nucleic acids, and proteins. Paper chromatography is used in the separation of proteins, and in studies related to protein synthesis; gas chromatography is utilized in the separation of alcohol, ester, lipid, and amino groups, and monitoring of enzymatic reactions. The chromatography based on permeation is employed especially for the determination of molecular weights of proteins.
Generally, chromatography techniques can be divided into two large classes depending on the mobile phase:
- Gas chromatography: the mobile phase is a gas, such as Helium.
- Liquid chromatography: the mobile phase is a liquid, such as acetonitrile or a buffer
There are two types of stationary phase supports:
- Column: In column chromatography, the stationary phase is packed into a column and the mobile phase flows through
- Planar layer: In planar layer chromatography, the stationary phase is on a flat plate or in the pores of paper, and the mobile phase moves by capillary action.
Figure 2 shows classification of chromatographic methods based on mechanism of separation as well as mobile and stationary phases used for separation.
C. Different Types of Chromatography
1. Ion exchange chromatography
Ion- exchange chromatography is based on electrostatic interactions between charged analyte groups, and solid support material (matrix). Matrix has an ion load opposite to that of the analyte to be separated, and the affinity of the analyte to the column is achieved with ionic interaction. Analytes are separated from the column either by changing pH, concentration of ion salts or ionic strength of the buffer solution. Positively charged ion- exchange matrices are called anion-exchange matrices, and adsorb negatively charged analytes. While matrices bound with negatively charged groups are known as cation-exchange matrices, and adsorb positively charged analytes.
2. Gel permeation (molecular sieve) chromatography
The basic principle of this method is to use dextran containing materials to separate macromolecules based on their differences in molecular sizes. This procedure is basically used to determine molecular weights of proteins, and to decrease salt concentrations of protein solutions. In a gel- permeation column stationary phase consists of inert molecules with small pores. The solution containing molecules of different dimensions are passed continuously with a constant flow rate through the column. Molecules larger than pores can not permeate into gel particles, and they are retained between particles within a restricted area. Larger molecules pass through spaces between porous particles, and move rapidly through inside the column. Molecules smaller than the pores are diffused into pores, and as molecules get smaller, they leave the column with proportionally longer retention times. Sephadex G type is the most frequently used column material. Besides, dextran, agorose, polyacrylamide are also used as column materials.
3. Affinity chromatography
Affinity Chromatography is used for the purification of enzymes, hormones, antibodies, nucleic acids, and specific proteins. A ligand which can form a complex with specific protein (dextran, polyacrylamide, cellulose etc) binds the filling material of the column. The specific protein which makes a complex with the ligand is attached to the solid support (matrix), and retained in the column, while free proteins leave the column. Then the bound protein leaves the column by means of changing its ionic strength through alteration of pH or addition of a salt solution.
4. Paper chromatography
In paper chromatography support material consists of a layer of cellulose highly saturated with water. In this method a thick filter paper comprised the support, and water drops settled in its pores made up the stationary “liquid phase.” Mobile phase consists of an appropriate fluid placed in a developing tank. Paper chromatography is a “liquid-liquid” chromatography.
5. Thin layer chromatography
Thin layer chromatography is a “solid-liquid adsorption” chromatography. In this method stationary phase is a solid adsorbent substance coated on glass plates. As adsorbent material all solid substances used. in column chromatography (alumina, silica gel, cellulose) can be utilized. In this method, the mobile phase travels upward through the stationary phase. The solvent travels up the thin plate soaked with the solvent by means of capillary action. During this procedure, it also drives the mixture priorly dropped on the lower parts of the plate with a pipette upwards with different flow rates. Thus the separation of analytes is achieved. This upward travelling rate depends on the polarity of the material, solid phase, and of the solvent.
In cases where molecules of the sample are colorless, fluorescence, radioactivity, or a specific chemical substance can be used to produce a visible colored reactive product so as to identify their positions on the chromatogram. Formation of a visible color can be observed under room light or UV light. The position of each molecule in the mixture can be measured by calculating the ratio between the the distances travelled by the molecule and the solvent. This measurement value is called relative mobility, and expressed with a symbol Rf. Rf. value is used for qualitative description of the molecules.
6. Gas chromatography
In gas chromatography, stationary phase is a column which is placed in the device, and contains a liquid stationary phase which is adsorbed onto the surface of an inert solid. Gas chromatography is a “gas-liquid” chromatography. Its carrier phase consists of gases as He or N2. Mobile phase which is an inert gas is passed through a column under high pressure. The sample to be analyzed is vaporized, and enters into a gaseous mobile phase phase. The components contained in the sample are dispersed between mobile phase, and stationary phase on the solid support. Gas chromatography is a simple, multifaceted, highly sensitive, and rapidly applied technique for the extremely excellent separation of very minute molecules. It is used in the separation of very little amounts of analytes.
7. Hydrophilic interaction liquid chromatography (HILIC)
Present theory proposes that HILIC retention is caused by partitioning. In this mode, the separation mechanism is based on the differential distribution of the injected analyte solute molecules between the acetonitrile-rich mobile phase and a water-enriched layer adsorbed onto the hydrophilic stationary phase. HILIC used for the analysis of charged analytes.
8. Hydrophobic interaction chromatography (HIC)
In this method, the adsorbents prepared as column material for the ligand binding in affinity chromatography are used. HIC technique is based on hydrophobic interactions between side chains bound to chromatography matrix.
9. High performance liquid chromatography (HPLC)
HPLC is not a new technique but rather an advancement. Using this chromatography technique it is possible to perform structural, and functional analysis, and purification of many molecules within a short time, This technique yields perfect results in the separation, and identification of amino acids, carbohydrates, lipids, nucleic acids, proteins, steroids, and other biologically active molecules, In HPLC, mobile phase passes through columns under 10–400 atmospheric pressure, and with a high (0.1–5 cm//sec) flow rate. In this technique, use of small particles, and application of high pressure on the rate of solvent flow increases separation power, of HPLC and the analysis is completed within a short time. HPLC routinely uses gradient elution i.e. increasing elution strength mobile phase over time for separation of different analyte molecules.
Essential components of a HPLC device are solvent compartment, high- pressure pump, commercially prepared column, detector, and recorder. Duration of separation is controlled with the aid of a computerized system.
10. Immobilized metal affinity chromatography
Immobilized metal affinity chromatography (IMAC) is a specialized variant of affinity chromatography where the proteins or peptides are separated according to their affinity for metal ions that have been immobilized by chelation to an insoluble matrix. At pH values around neutral, the amino acids histidine, tryptophan, and cysteine form complexes with the chelated metal ions (e.g., Zn2+, Cu2+, Cd2+, Hg2+, Co2+, Ni2+, and Fe2+). They can then be eluted by reducing the pH, increasing the mobile phase ionic strength, or adding ethylenediaminetetraacetic acid to the mobile phase. Metal chelate affinity chromatography is excellent for purifying recombinant (His)6 fusion proteins as well as many natural proteins.
11. Hyphenated Techniques
Hyphenation of different gas and liquid chromatography techniques results in very powerful analytical tool. Some examples of hyphenation are GC-MS (gas chromatography – mass spectrometry), LC-MS (liquid chromatography – mass spectrometry). While mass spectrometry can identify analytes by two physical properties, precursor and product ion masses, another property is added when used in tandem with liquid chromatography to identify the analyte even more accurately. Liquid chromatography and mass spectrometry also provide the ability to multiplex, or the ability to identify and quantify several analytes at once.
D. Chromatography application areas
1. Pharmaceutical and Clinical Testing
Pharmaceutical analysis is one of the major applications of chromatography. Pharmaceutical companies use chromatography to quantify and analyze compounds for contaminants. For example, chiral compounds have two different forms due to their atoms differing slightly in space. One form of chiral compounds is known to be toxic. Chromatography can ensure that the safe form is separate from the dangerous form of the chiral compound. Vaccine development is also an application of chromatography. In tandem with mass spectrometry, liquid chromatography has revolutionized the clinical laboratory testing.
2. Food and Beverage
Quality control within the food and beverage industry can be enacted through chromatography. In the food industry, chromatography is used to separate and analyze additives, vitamins, proteins, amino acids, and other nutritional compounds in food items. Chromatography can also be used to determine expiration dates by distinguishing the number of organic acids present as well as to detect any harmful toxins that may have been added to the food item. In the beverage industry specifically, chromatography can be used to make sure every bottle of a drink prepared is consistent. For example, chromatography can separate a soda mixture to ensure every can has the same sugar content, keeping each bottle consistent in taste.
3. Environmental and Chemical Industry
The chemical industry must adhere to numerous environmental safety precautions. Perfluoroalkyl substances, also known as PFAS, have become a persistent threat to the human body and the environment. PFAS can be found in items such as protective coatings on shoes and other fabrics, electronics, and even firefighting foams. While these substances benefit products by making them extremely durable, they pose an environmental concern as they continue to accumulate. PFAS in our drinking water can also lead to damaging health concerns such as reproductive and developmental setbacks. By using solid-phase extraction, liquid chromatography, and mass spectrometry, we can detect PFAS in the environment and our drinking water, even at very low limits.
4. Drug Testing
Chromatography can be very useful in drug testing and clinical toxicology reports. Chromatography can separate and analyze substances found in urine samples. When running a clinical toxicology report, drug testing a new employee or testing a professional athlete for performance-enhancing drugs, chromatography determines what substances have been taken through an analysis of a urine sample which ultimately determines if any harmful or illicit drugs have been used.
5. Security
Security practices are a unique industry where chromatography can be utilized. Gas chromatography can be used to determine volatile gases, furthering safety measures at locations such as airports and large gatherings with similar safety precautions like concerts and sporting events to eliminate deadly threats.
6. Forensics
Forensics is a key application of chromatography. Gas chromatography can be used for more in-depth forensics procedures, for example, crime scene analysis to test evidence such as blood, hair, and fabric samples to further understand what may have happened at the scene. Chromatography is massively important to forensic pathology work. Gas chromatography is widely used to identify the types of fluids and compounds that exist in a body postmortem. In such cases, a possible cause of death and motive can be determined based on finding drugs, alcohol, or toxic substances in the body. Another unique form of forensics that can be assisted by chromatography is arson verification. By using chromatography in arson verification, it’s possible to identify flammable substances in fire debris to determine the exact substance that created the fire.
7. Molecular Biology Studies
One of the most complex uses of chromatography is molecular biology studies. Hybrid techniques between electrochemistry (EC) and mass spectrometry with chromatography are often applied to studies of proteins, peptides, and nucleic acids. This combination is largely used for metabolomics such as biotransformation reactions like oxidative reactions and proteomics such as the purification of plasma proteins, hormones, and antibodies. Chromatography in nucleic acid research plays a role in accelerating the identification process of nucleobases, nucleotides, and nucleosides, as well as identifying their oxidization process.
8. Petroleum
Gas chromatography is used to analyze finished gas products and refining processes. Chromatography is most notably used in the analysis of natural and refinery gas for BTU content and hydrocarbon composition.
E. Summary
Chromatography is a versatile technique with important applications analytical, developmental, and quality control laboratories. Several different types of chromatography are used in several industries, including examples such as preparing safe pharmaceutical drugs and clinical testing, determining the expiration date and nutritional components of food and beverages, monitoring chemical safety impacts on the environment, and even aiding in forensics research. The various applications of chromatography all hold significant importance in their respective field to keep industries safe and to further understand changes in their landscape.
